
Publications
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
2017
2018
2019
2020
2021 2022
2023
2024
2025
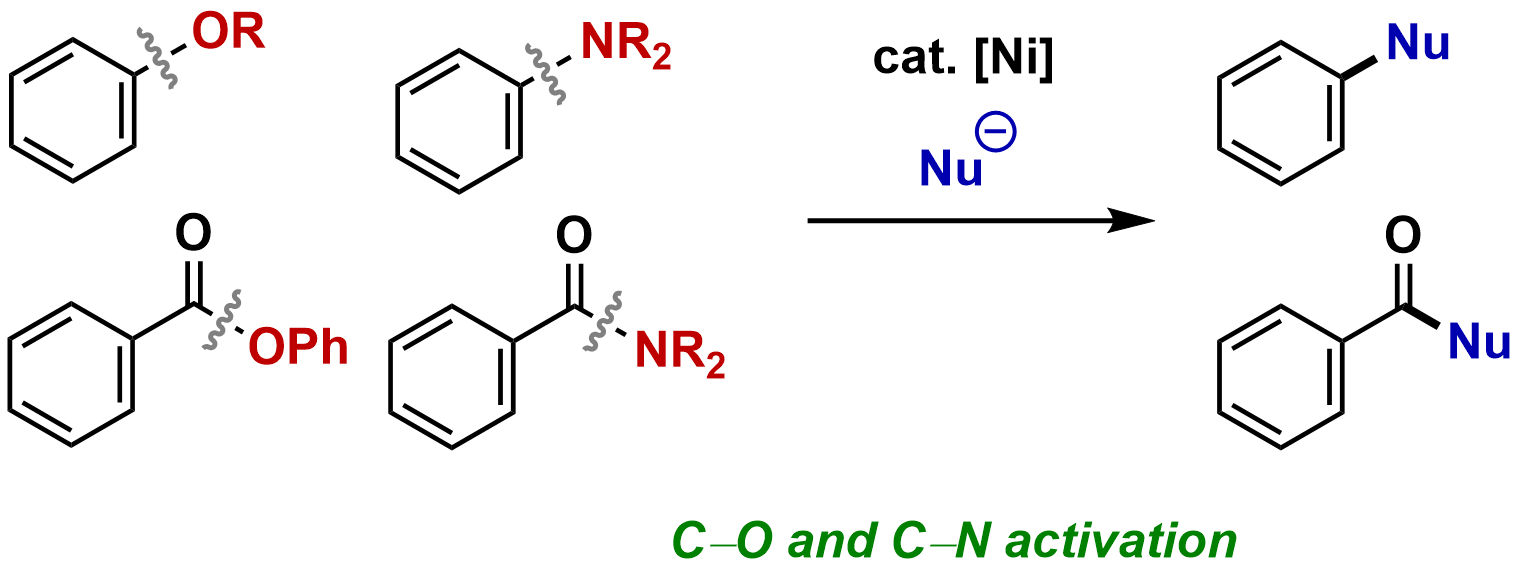
15. Nickel-Catalyzed Cross Coupling via C–O and C–N Activation

Tomoki Yoshida and Mamoru Tobisu
Science of Synthesis: Base-Metal Catalysis, 2022, 1, 591–630.
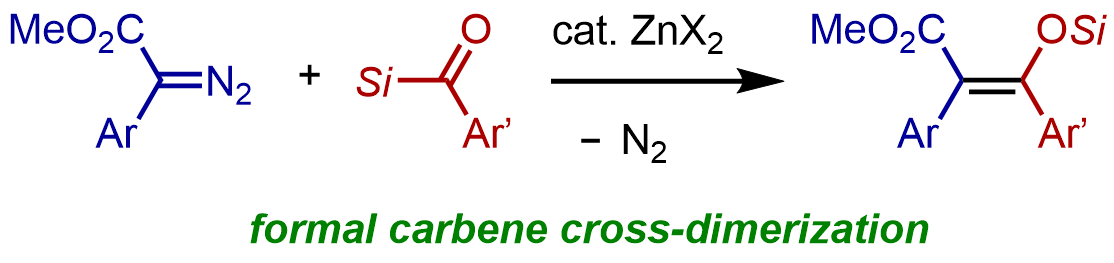
14. Zn(II)-catalyzed Formal Cross-Dimerization of Carbenes Using Acylsilanes and Diazo Esters
Tomoki Yoshida, Masaya Ohta, Tomoya Emmei, Takuya Kodama, and Mamoru Tobisu
Chem. Lett., 2023, 52(1), 48–50.
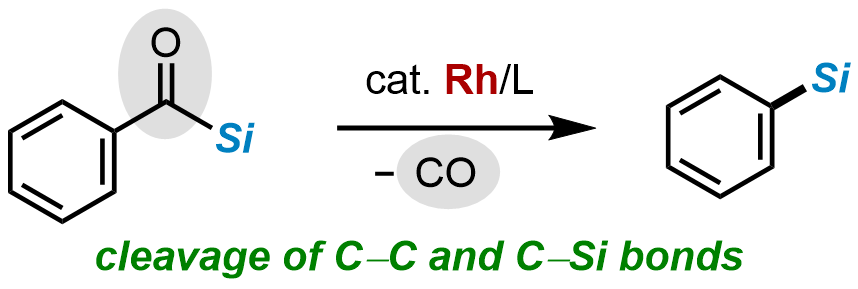
13. Rhodium-catalyzed Decarbonylation of Acylsilanes
Tomoki Yoshida, Takuya Kodama, and Mamoru Tobisu
Asian J. Org. Chem., 2022, 11(12), e202200610. (Invited contribution to the special collection on the occasion of Professor Keiji Maruoka's 70th birthday)
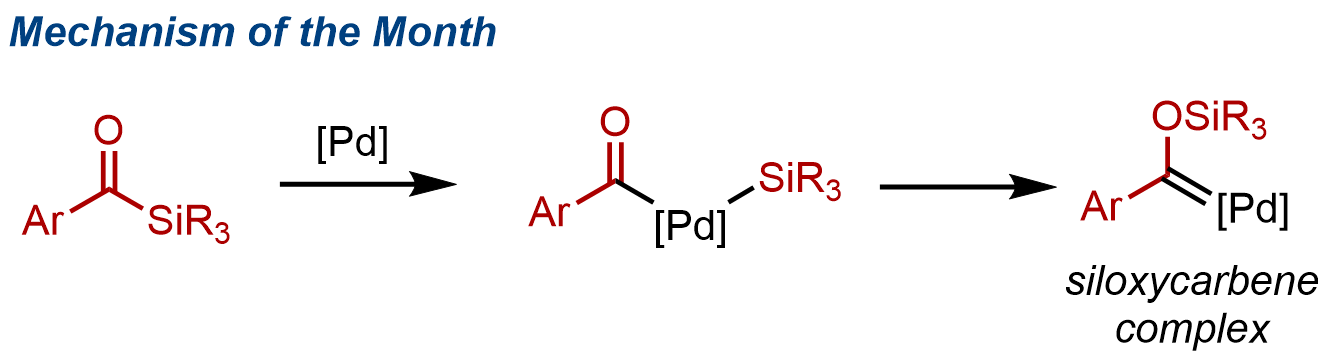
12. Pd-catalyzed siloxycyclopropanation of alkenes
Shun Sakurai, Tetsuya Inagaki, Takuya Kodama, Masahiro Yamanaka, and Mamoru Tobisu
Trends in Chemistry, 2022, 4(12), 1161–1162. (Invited contribution to 'Mechanism of the Month')
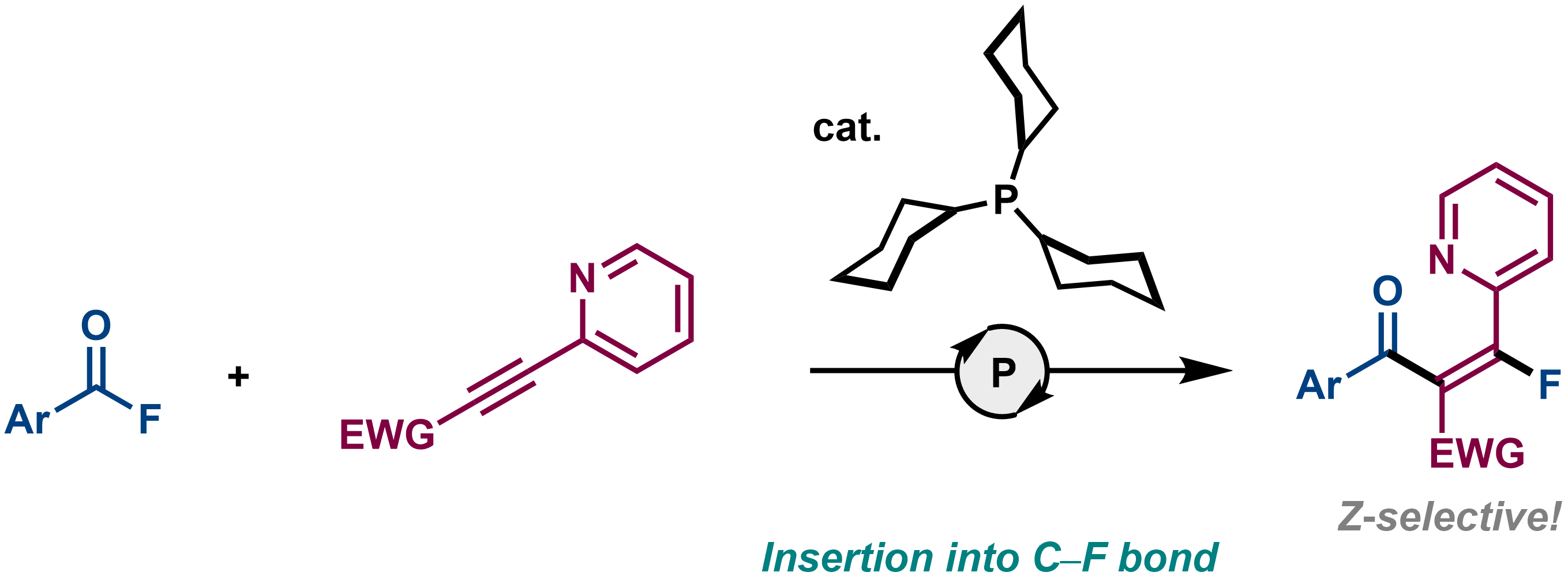
11. Phosphine-Catalyzed Z-Selective Carbofluorination of Alkynoates Bearing an N-Heteroarene Units
Hayato Fujimoto, Shisato Yamamura, Namiki Takenaka, and Mamoru Tobisu
Synthesis, 2022, in press (Invited contribution to the special issue on 'Synthetic Advancements Enabled by Phosphorus Redox Chemistry')
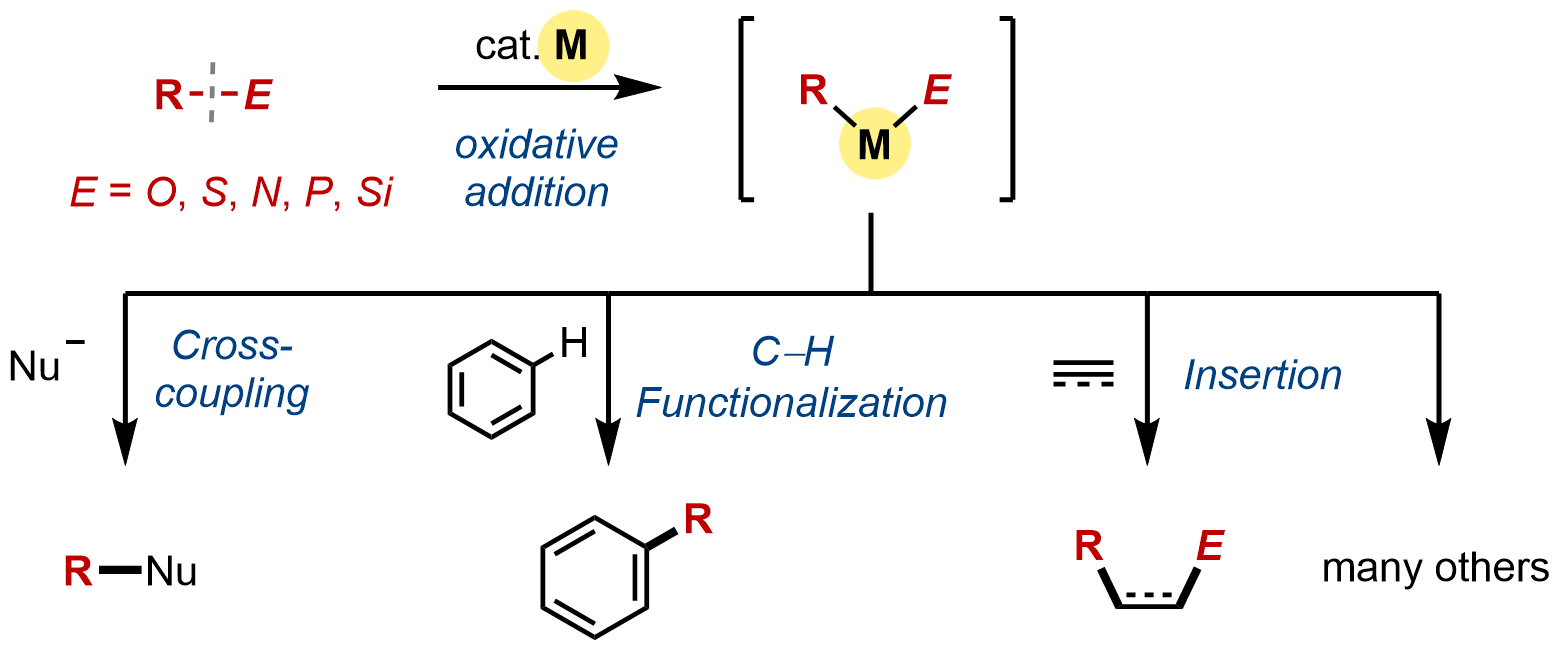
10. Synthetic Applications of C–O and C–E Bond Activation Reactions

Mamoru Tobisu, Takuya Kodama and Hayato Fujimoto
In Comprehensive Organometallic Chemistry IV, 2022, 12, pp347–420.
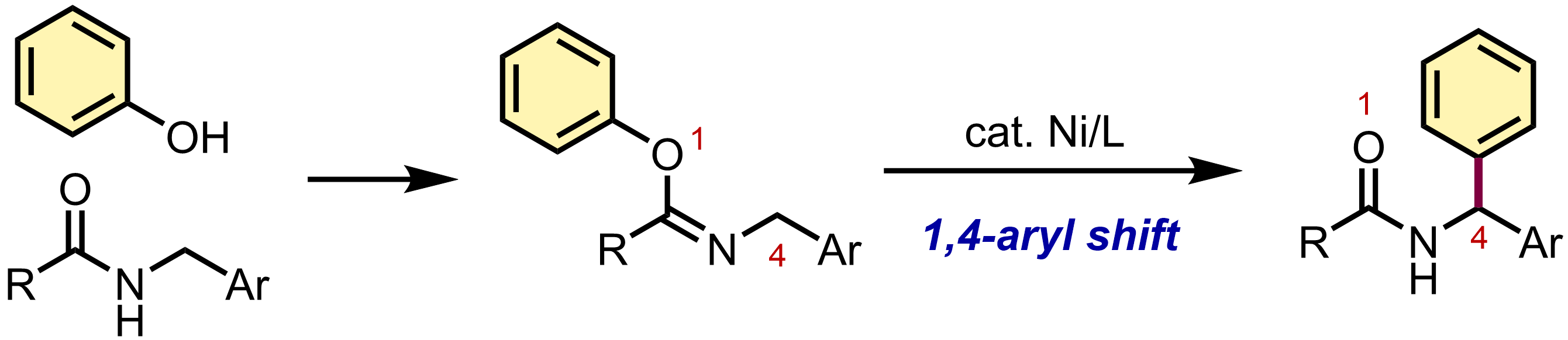
9. Nickel-catalyzed 1,4-aryl rearrangement of aryl N-benzylimidates via C–O and C–H bond cleavage

Satoshi Ogawa and Mamoru Tobisu
Chem. Commun., 2022, 58(57), 7909–7911.
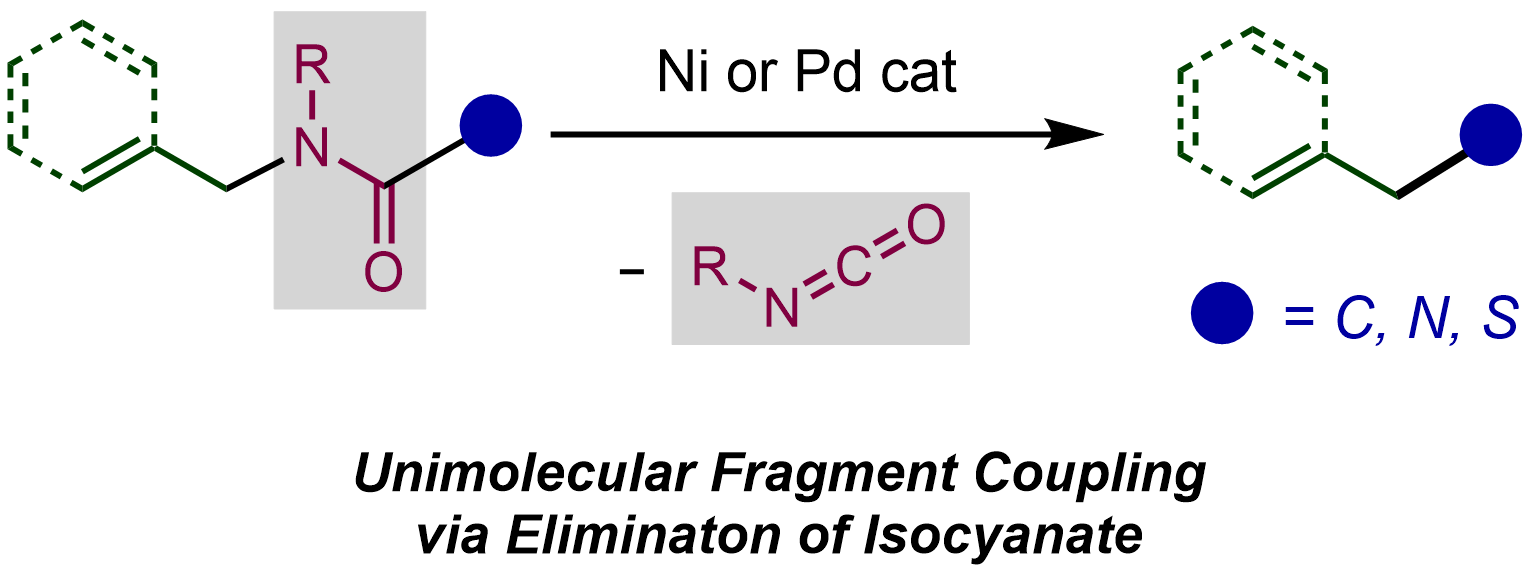
8. Palladium-Catalyzed Unimolecular Fragment Coupling of N-Allylamides via Elimination of Isocyanate
Ryoma Shimazumi, Riku Tanimoto, Takuya Kodama, and Mamoru Tobisu
J. Am. Chem. Soc., 2022, 144(24), 11033–11043.
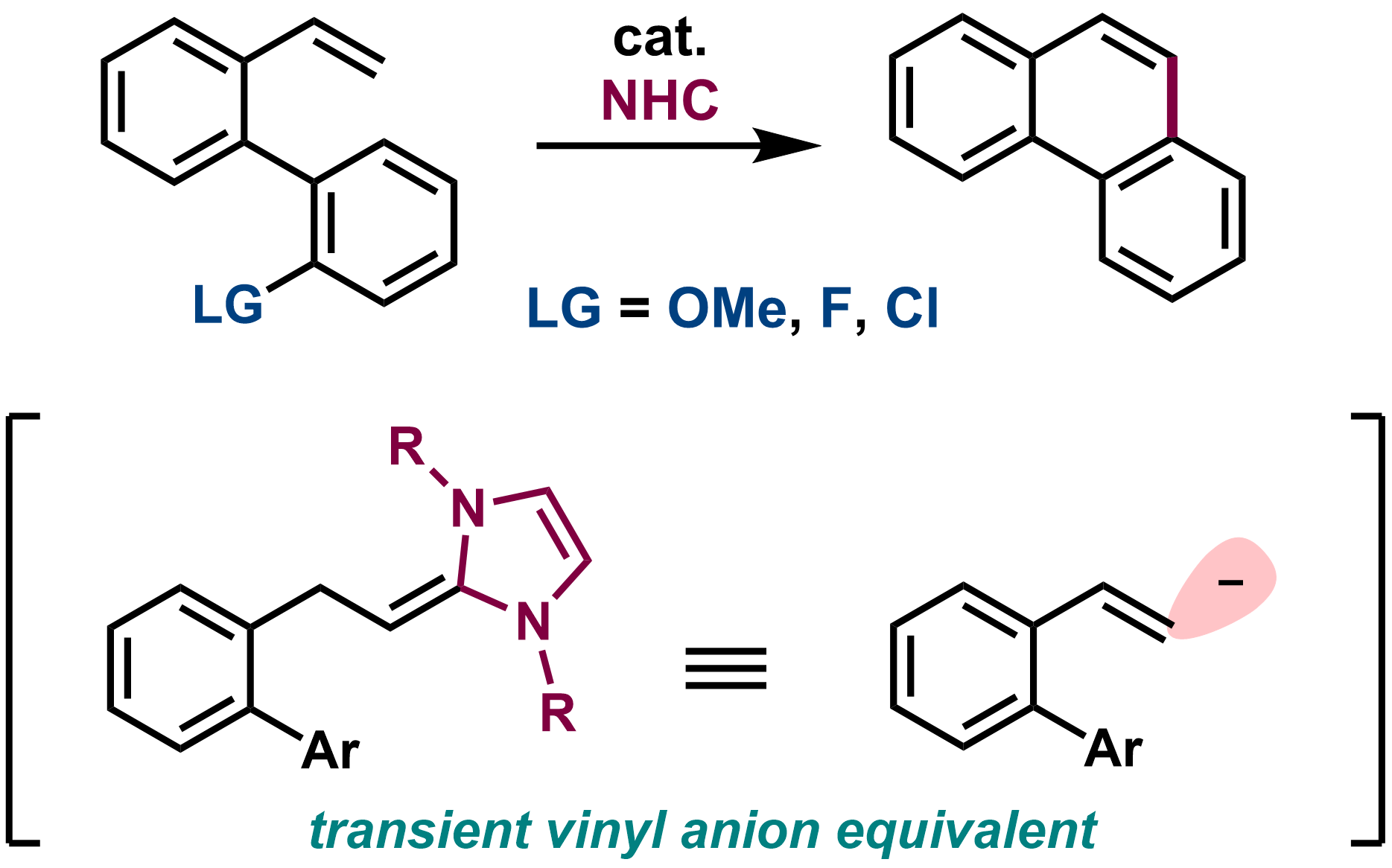
7. Non-Stabilized Vinyl Anion Equivalents from Styrenes by N-Heterocyclic Carbene Catalysis and Its Use in Catalytic Nucleophilic Aromatic Substitution
Sora Ito, Hayato Fujimoto, and Mamoru Tobisu
J. Am. Chem. Soc., 2022, 144(15), 6714–6718.
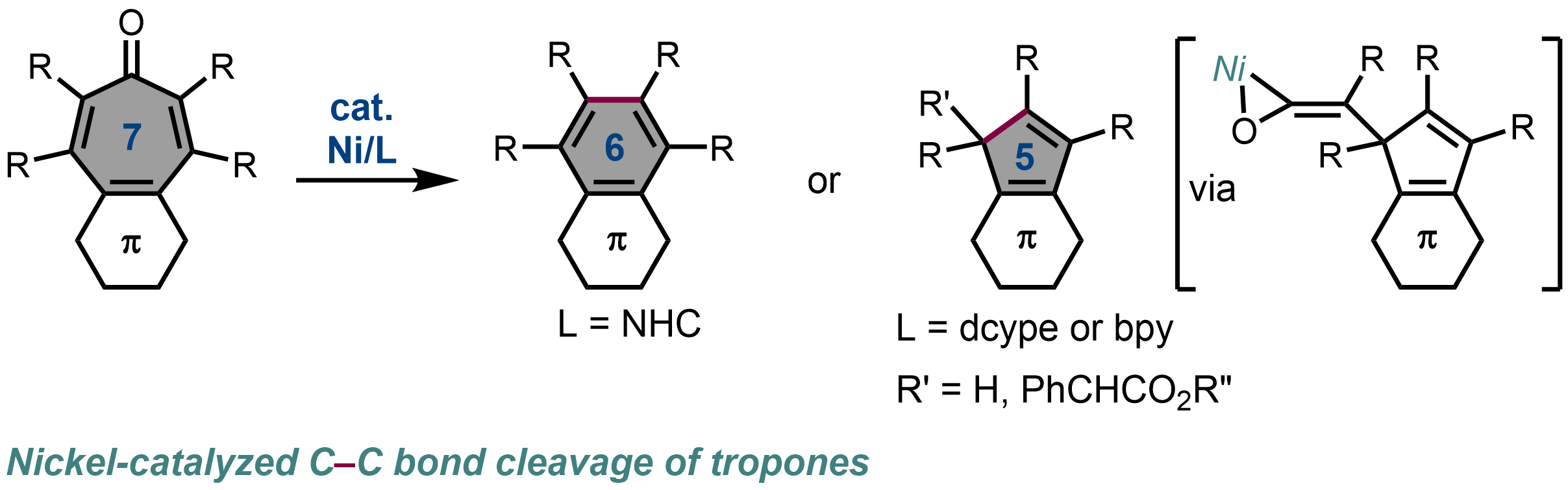
6. Nickel-Catalyzed Skeletal Transformation of Tropone Derivatives via C–C Bond Activation: Catalyst-Controlled Access to Diverse Ring Systems
Takuya Kodama, Kanako Saito, and Mamoru Tobisu
Chem. Sci., 2022, 13(17), 4922–4929.
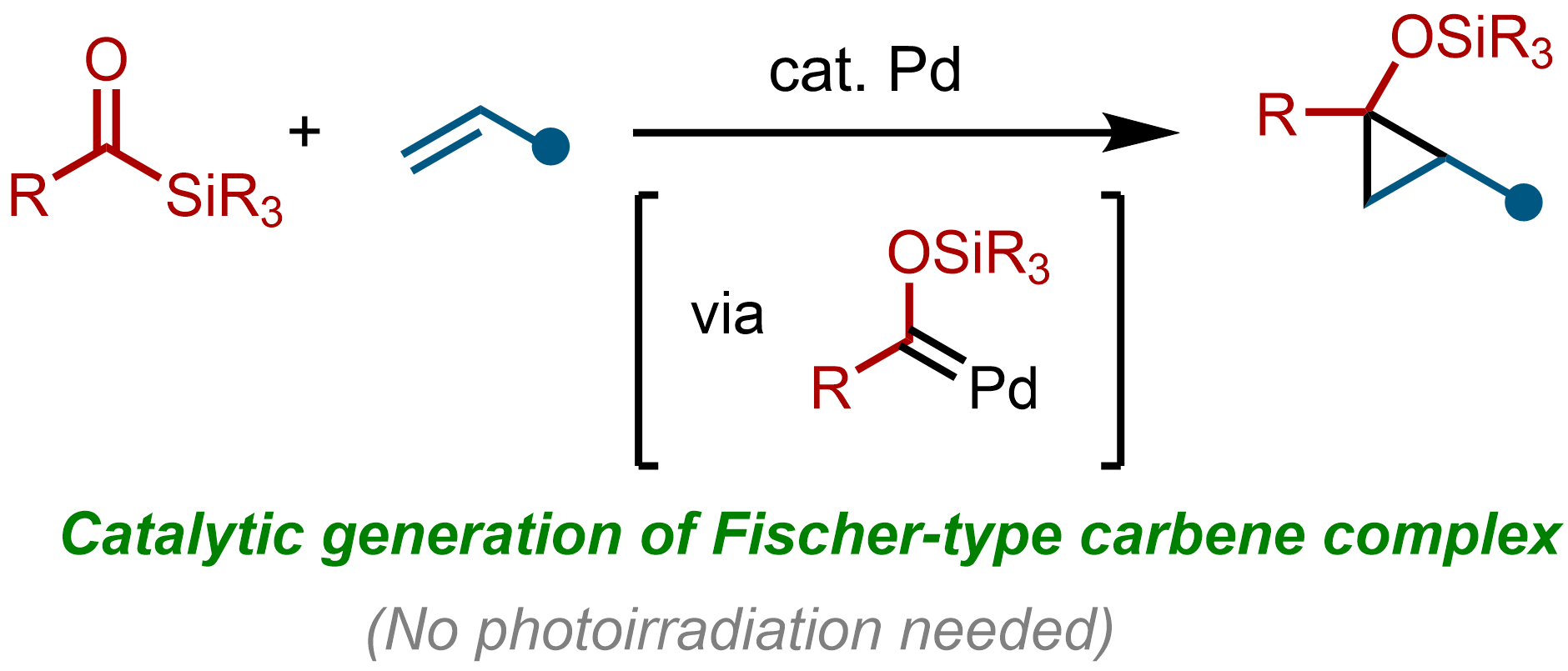
5. Palladium-Catalyzed Silylacylation of Allenes Using Acylsilanes
Tetsuya Inagaki, Shun Sakurai, Masahiro Yamanaka, and Mamoru Tobisu
Angew. Chem. Int. Ed., 2022, 61(21), e202202387.

4. Overlooked Factors Required for Electrolyte Solvents in Li–O₂ Batteries: Capabilities of Quenching 1O₂ and Forming Highly-Decomposable Li₂O₂
Kiho Nishioka, Mizuki Tanaka, Hayato Fujimoto, Toru Amaya, Sensuke Ogoshi, Mamoru Tobisu, and Shuji Nakanishi
Angew. Chem. Int. Ed., 2022, 61(12), e202112769.
3. Ratiometric and colorimetric detection of Cu2+ via the oxidation of benzodihydroquinoline derivatives and related synthetic methodology
Waroton Paisuwan, Vachiraporn Ajavakom, Mongkol Sukwattanasinitt, Mamoru Tobisu, Anawat Ajavakom
Sens. Bio-Sens. Res., 2022, 35, 100470.
2. Palladium-Catalyzed Siloxycyclopropanation of Alkenes Using Acylsilanes
Shun Sakurai, Tetsuya Inagaki, Takuya Kodama, Masahiro Yamanaka, and Mamoru Tobisu
J. Am. Chem. Soc., 2022, 144(3), 1099–1105.
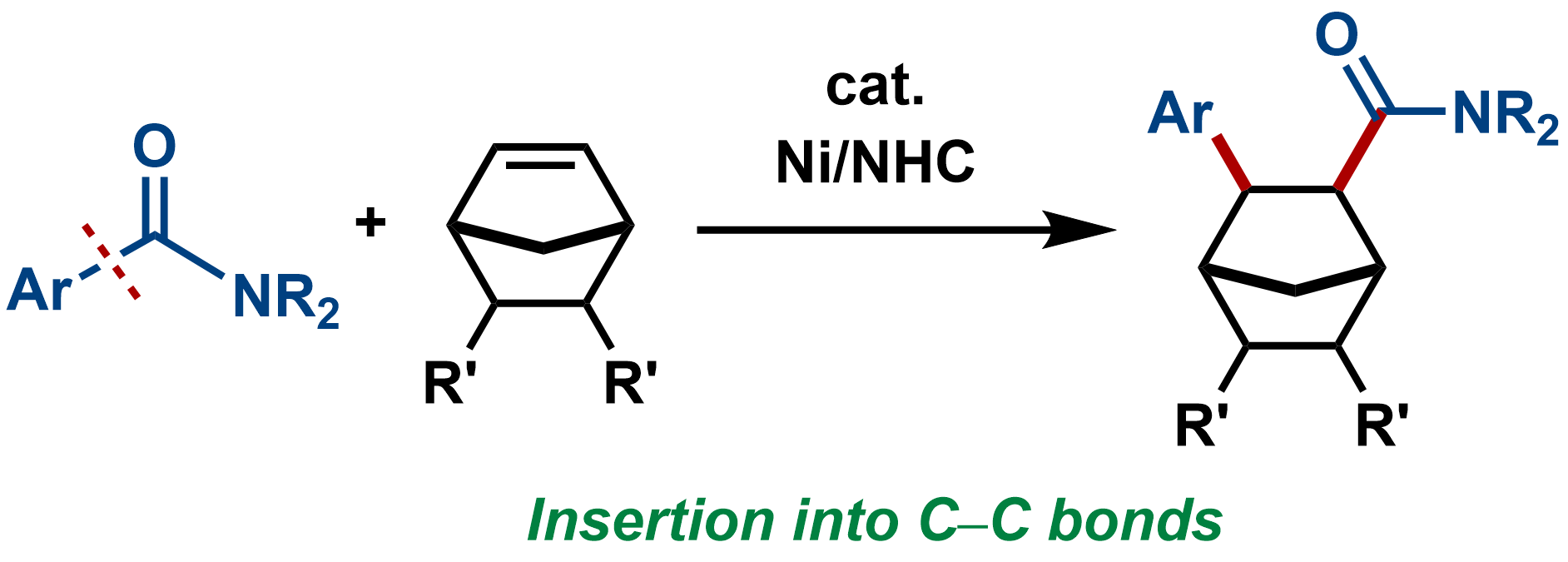
1. Nickel-Catalyzed Addition of C–C Bonds of Amides to Strained Alkenes: The 1,2-Carboaminocarbonylation Reaction
Yuri Ito, Syun Nakatani, Ryota Shiraki, Takuya Kodama, and Mamoru Tobisu
J. Am. Chem. Soc., 2022, 144(2), 662–666.
Copyright(c)All right reserved. The Tobisu Group.





