
Publications
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
2017
2018 2019
2020
2021
2022
2023
2024
2025
15. Novel Development of Umpolung at Main Group Element: Synthesis, Structure and Reactivity of Nucleophilic Aluminyl Anion
Takuya Kodama
J. Synth. Org. Chem. Jpn. 2019, 77(12), 1247–1249.(Review de Debut)
14. Oxovanadium(V)-catalyzed deoxygenative homocoupling reaction of alcohols
Takashi Sakuramoto, Yosuke Donaka, Mamoru Tobisu, and Toshiyuki Moriuchi
New J. Chem., in press.
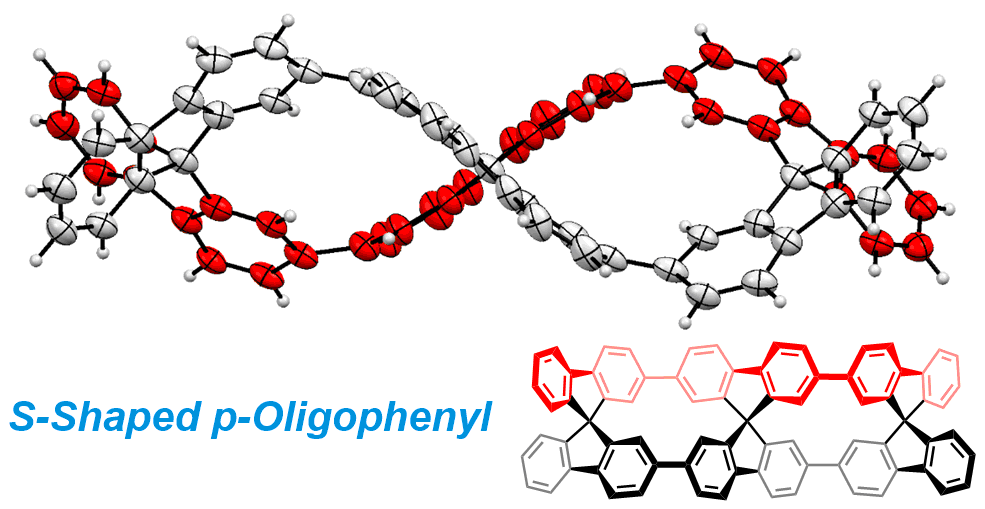
13. Linear [3]Spirobifluorenylene: An S-Shaped Molecular Geometry of p-Oligophenyls
Jumpei Oniki, Toshiyuki Moriuchi, Kosuke Kamochi, Mamoru Tobisu, and Toru Amaya
J. Am. Chem. Soc., 141 (45), 18238-18245 (2019).
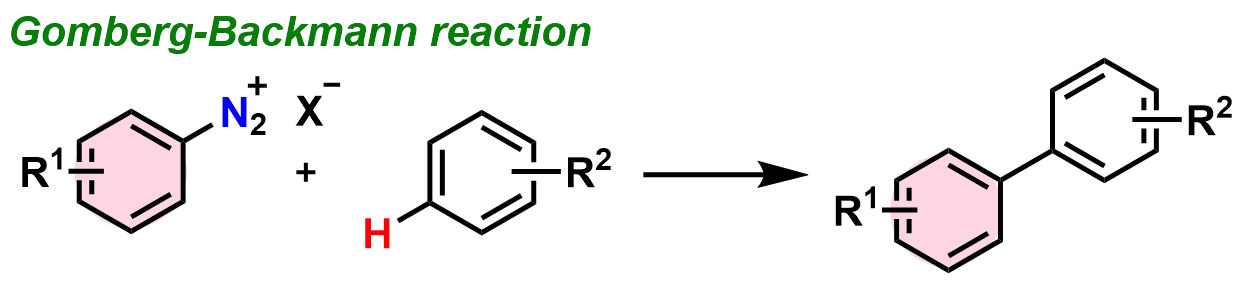
12. Recent Advances in Gomberg-Bachmann Biaryl Synthesis
Toru Amaya, Yuqing Jin, and Mamoru Tobisu
Tetrahedron Lett.., 60 (39), 151062 (2019). (Digest review).
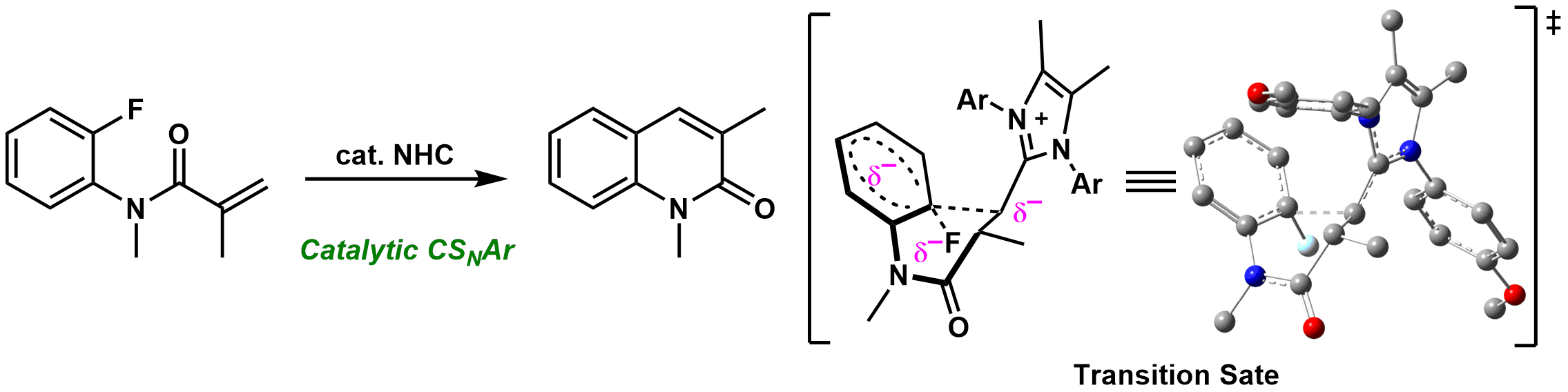
11. N]Heterocyclic Carbene]Catalyzed Concerted Nucleophilic Aromatic Substitution of Aryl Fluorides Bearing Ώ,ΐ]Unsaturated Amides
Kosuke Yasui, Miharu Kamitani, and Mamoru Tobisu
Angew. Chem. Int. Ed., 58 (40), 14157-14161 (2019).
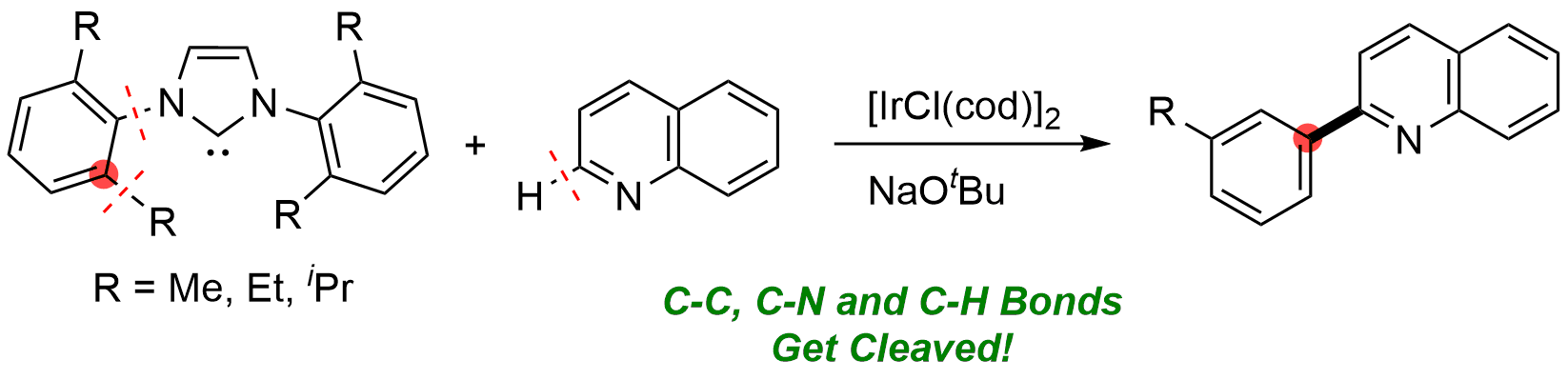
10. Iridium-Mediated Arylation of Quinoline via the Cleavage of Carbon-Carbon and Carbon-Nitrogen Bonds of 1,3-Dimesitylimidazol-2-ylidene
Shun Sakurai and Mamoru Tobisu
Organometallics, 38 (14), 2834-2838 (2019).
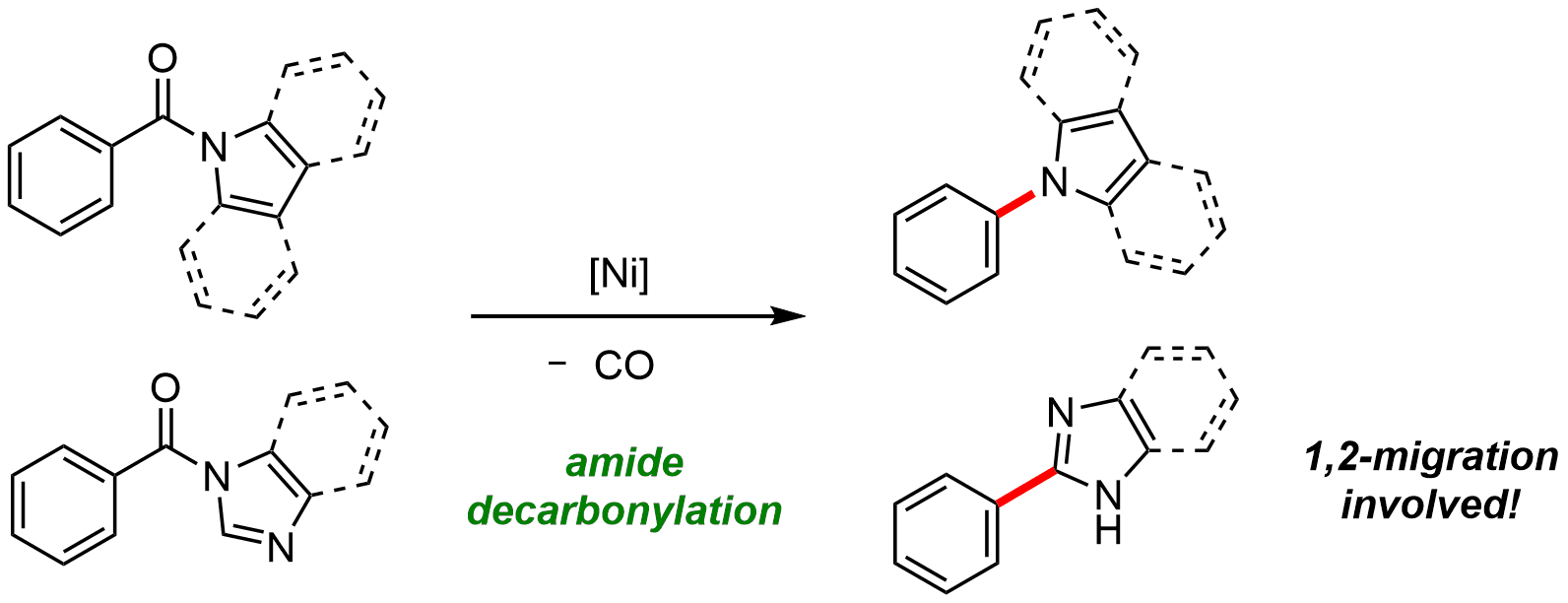
9. Nichel-Catalized Decarbonylation of N-Acylated N-Heteroarenes
Toshifumi Morioka, Syun Nakatani, Yuki Sakamoto, Takuya Kodama, Sensuke Ogoshi, Naoto Chatani, and Mamoru Tobisu
Chem. Sci., 10, 6666-6671 (2019).
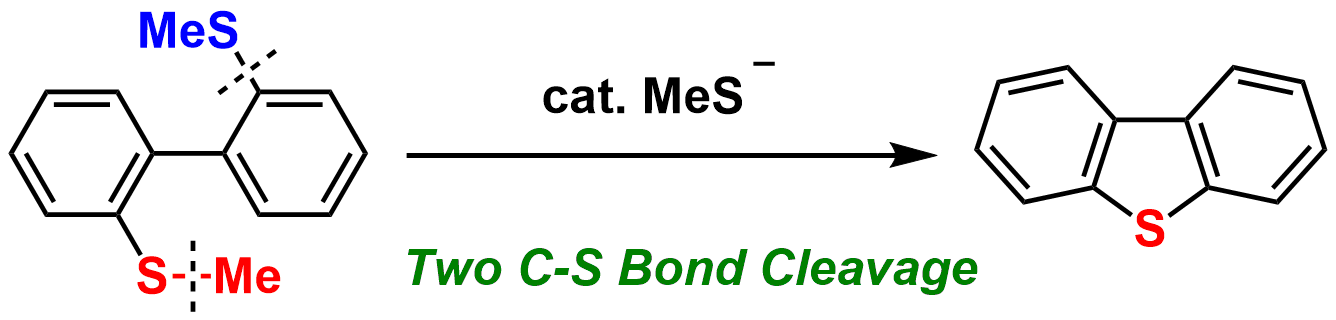
8. Thiolate-Induced Synthesis of Dibenzothiophenes from 2,2'-Bis(methylthio)-1,1'-Biar
Yoshihiro Masuya, Yuki Kawashima, Takuya Kodama, Naoto Chatani, and Mamoru Tobisu
Synlett, 30 (17), 1995-1999 (2019) (Invited contribution to Cluster on Metathesis).
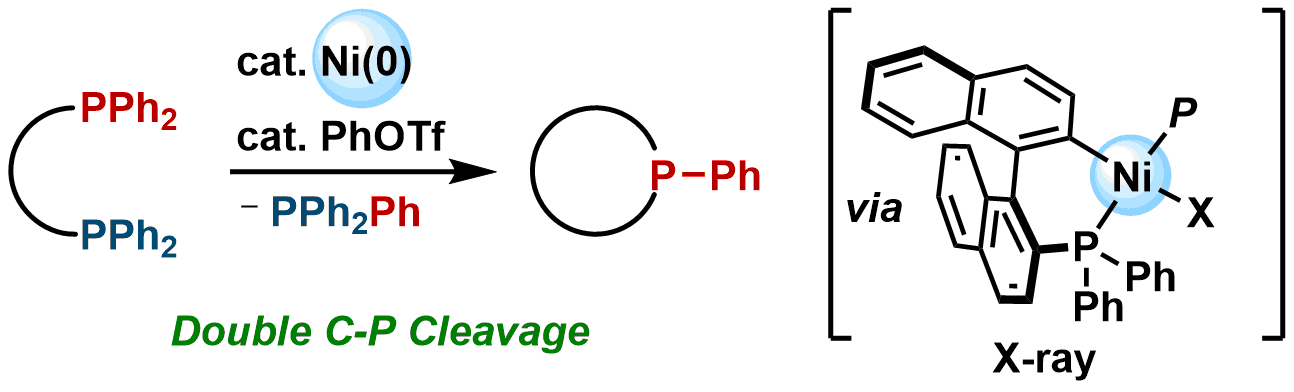
7. Cyclization of Bisphosphines to Phosphacycles via the Cleavage of Two Carbon–Phosphorus Bonds by Nickel Catalysis
Hayato Fujimoto, Momoka Kusano, Takuya Kodama, and Mamoru Tobisu
Org. Lett., 21(11) 4177-4181 (2019).
6. Nickel-Catalyzed Decarboxylation of Aryl Carbamates for Converting Phenols into Aromatic Amines
Akihiro Nishizawa, Tsuyoshi Takahira, Kosuke Yasui, Hayato Fujimoto, Tomohiro Iwai, Masaya Sawamura , Naoto Chatani, and Mamoru Tobisu
J. Am. Chem. Soc., 141(18) 7261-7265 (2019).
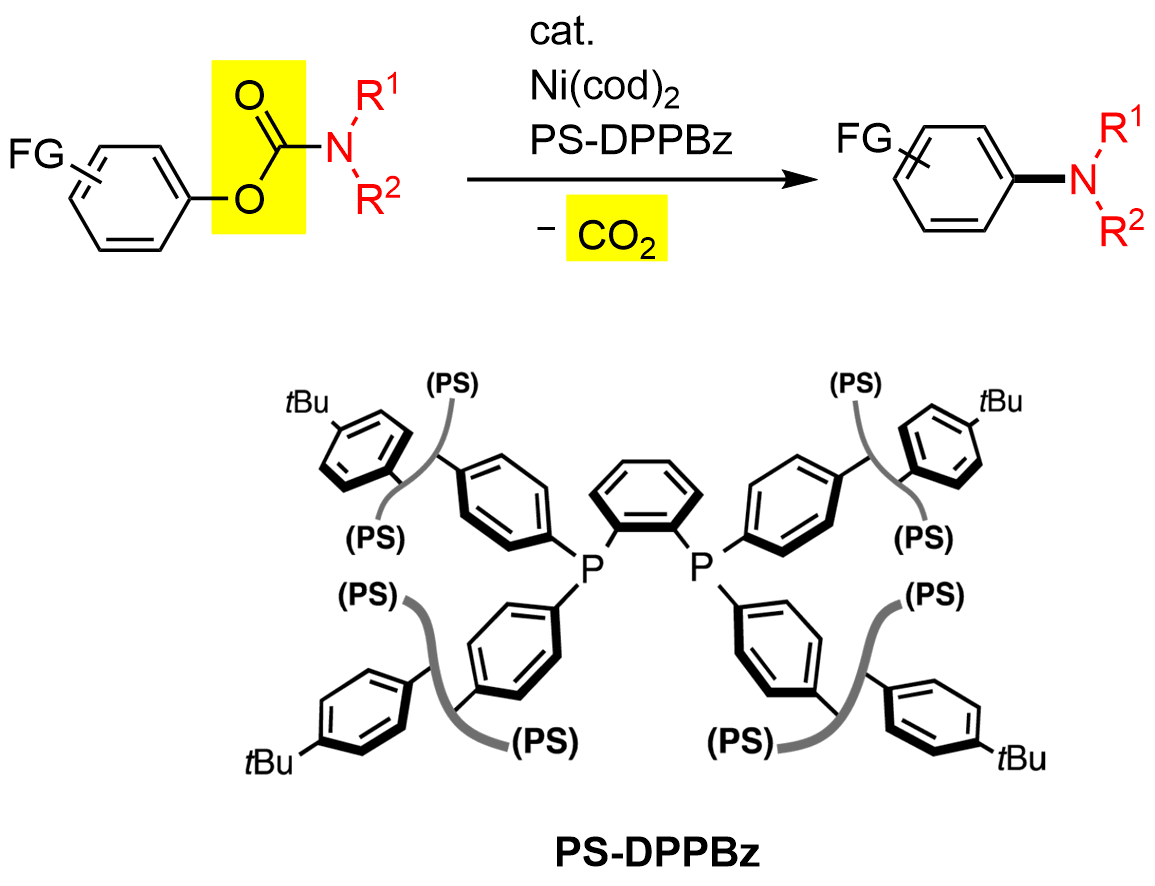
5. Synthesis of a Sumanenyl Hafnocene Complex
Toru Amaya, Shun Katoh, Toshiyuki Moriuchi, and Toshikazu Hirao
Org. Chem. Front., 6(7) 1032-1037 (2019).
This article is part of the themed collection: In celebration of Julius Rebekfs 75th Birthday.
4. Metal-Catalyzed Aromatic C-O Bond Activation/Transformation
Mamoru Tobisu
In Organometallics for Green Catalysis. Topics in Organometallic Chemistry, vol 63.; Pierre H. Dixneuf, Jean-Francois Soule Eds.; Springer: Cham, 2018; pp103-140.

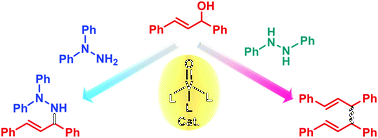
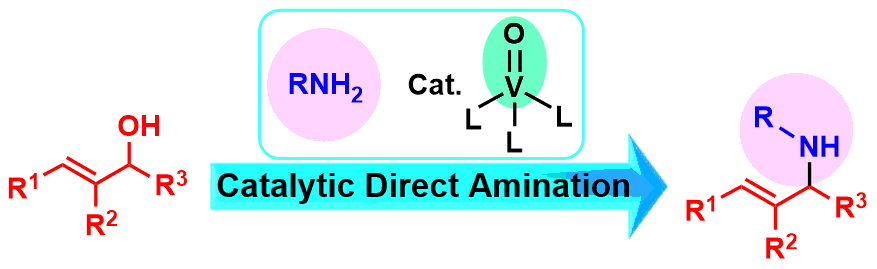
3. Oxovanadium(V)]Catalyzed Direct Amination of Allyl Alcohols
Takashi Sakuramoto, Toshikazu Hirao, Mamoru Tobisu,
ChemCatChem, 11(4), 1175-1178 (2019). (Selected as a "Cover Feature").
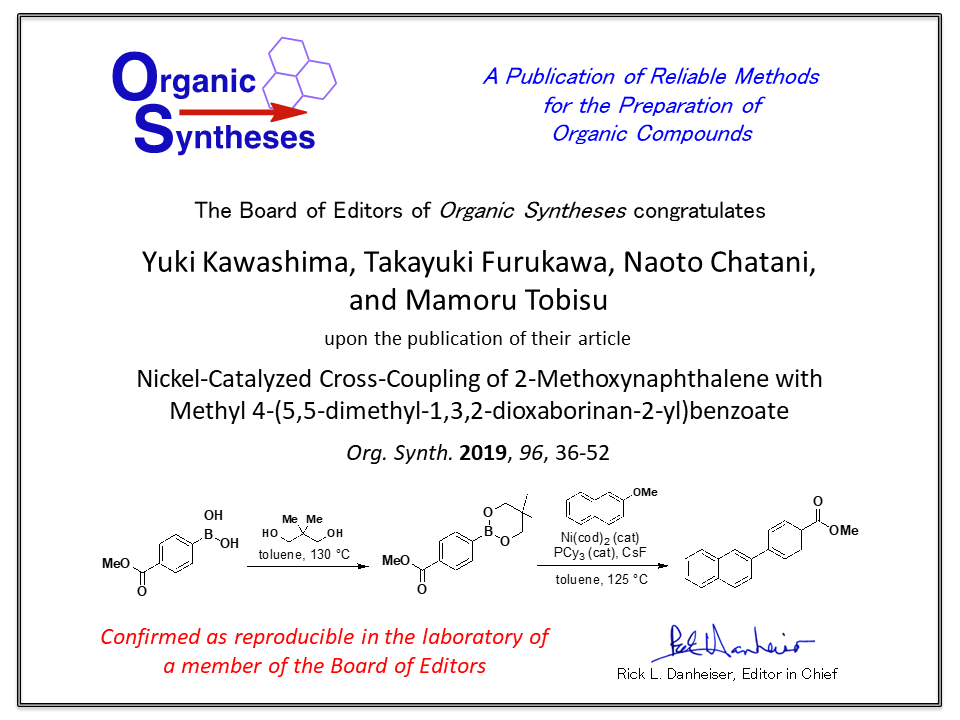
2. Nickel-Catalyzed Cross-Coupling of 2- Methoxynaphthalene with Methyl 4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)benzoate
Yuki Kawashima, Takayuki Furukawa, Naoto Chatani,
Org. Synth., 96, 36-52 (2019).

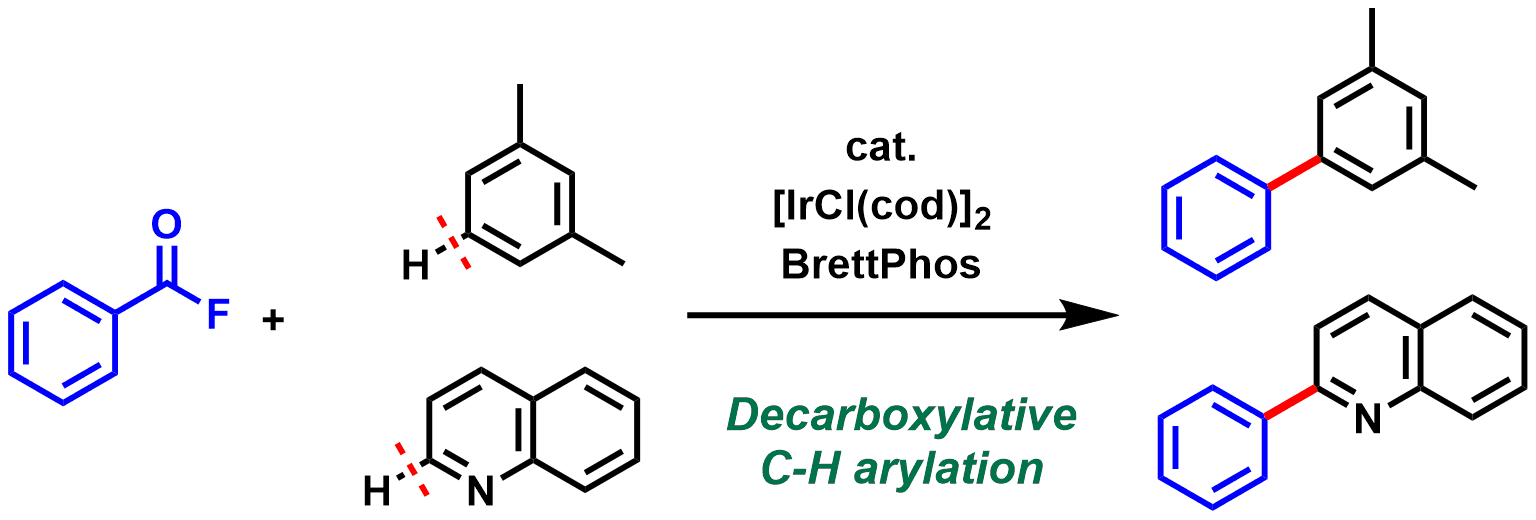
1. Iridium-catalyzed Decarbonylative Coupling of Acyl Fluorides with Arenes and Heteroarenes via C-H Activation
Chem. Lett., 48(1), 94-97 (2019).
Copyright(c)All right reserved. The Tobisu Group.





