2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 2026
-
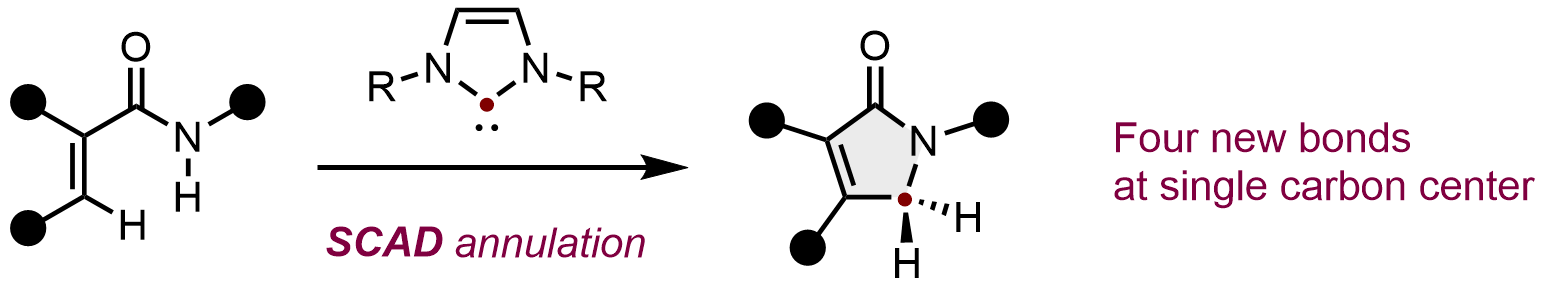
Synthesis of γ-Lactams from Acrylamides by Single-Carbon Atom Doping Annulation
Hayato Fujimoto, Bunta Nakayasu, and Mamoru Tobisu
J. Am. Chem. Soc. 2023, 145(36), 19518–19522.
DOI: https://pubs.acs.org/doi/full/10.1021/jacs.3c07052
-
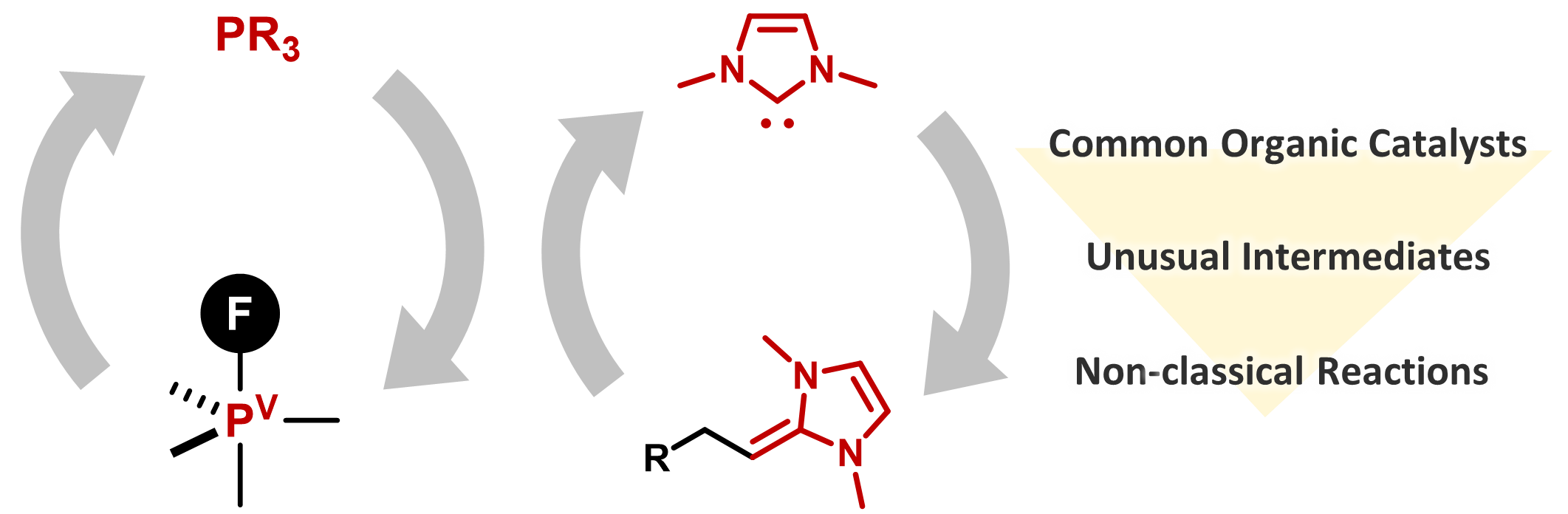
Non-classical Molecular Activation by Phosphines and N-Heterocyclic Carbenes and Its Application to Catalytic Reactions
Hayato Fujimoto, Kosuke Yasui, and Mamoru Tobisu
Bull. Chem. Soc. Jpn. 2023, 96(9), 872–886.
DOI: https://doi.org/10.1246/bcsj.20230150
-
Nickel/Photoredox Dual-Catalyzed Conversion of Allyl Esters to Ketones via the Formal Deletion of Oxygen
Ryoma Shimazumi, Riku Tanimoto and Mamoru Tobisu
Org. Lett. 2023, 25(34), 6440–6445.
DOI: hhttps://doi.org/10.1021/acs.orglett.3c02606

-
「ノックは不要」
鳶巣 守
ドラマチック有機合成化学, 有機合成化学協会編, pp. 90–91.

-
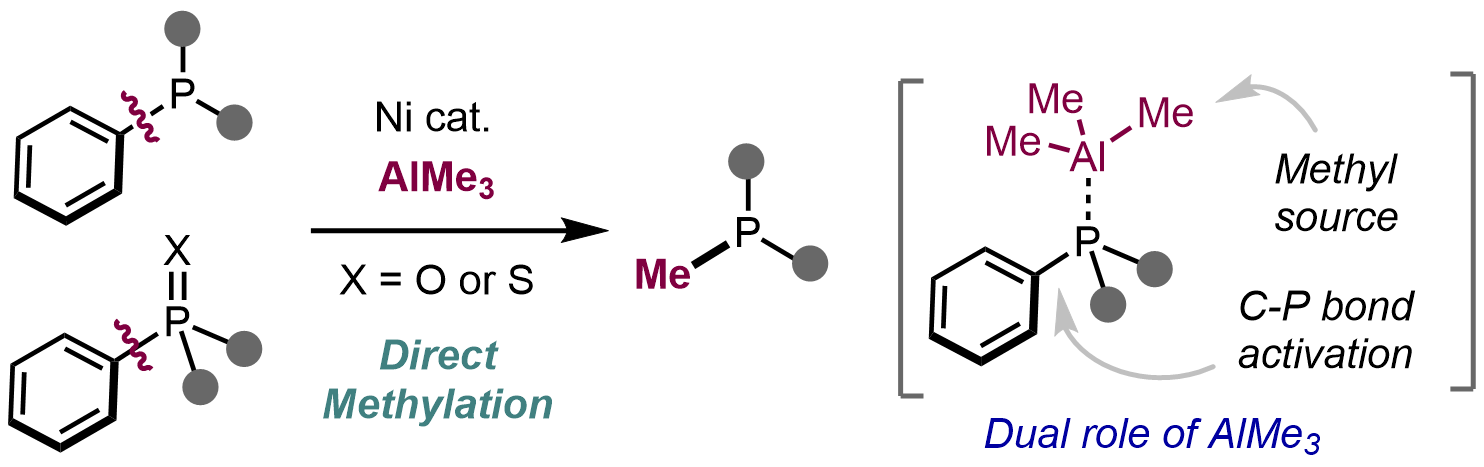
Nickel-catalyzed direct methylation of arylphosphines via carbon–phosphorus bond cleavage using AlMe3
Takuya Igarashi, Ryoma Shimazumi, Naoto Chatani and Mamoru Tobisu
Chem. Commun. 2023, 59(64), 9722–9725.
DOI: https://pubs.rsc.org/en/content/articlelanding/2023/CC/D3CC02455E
-
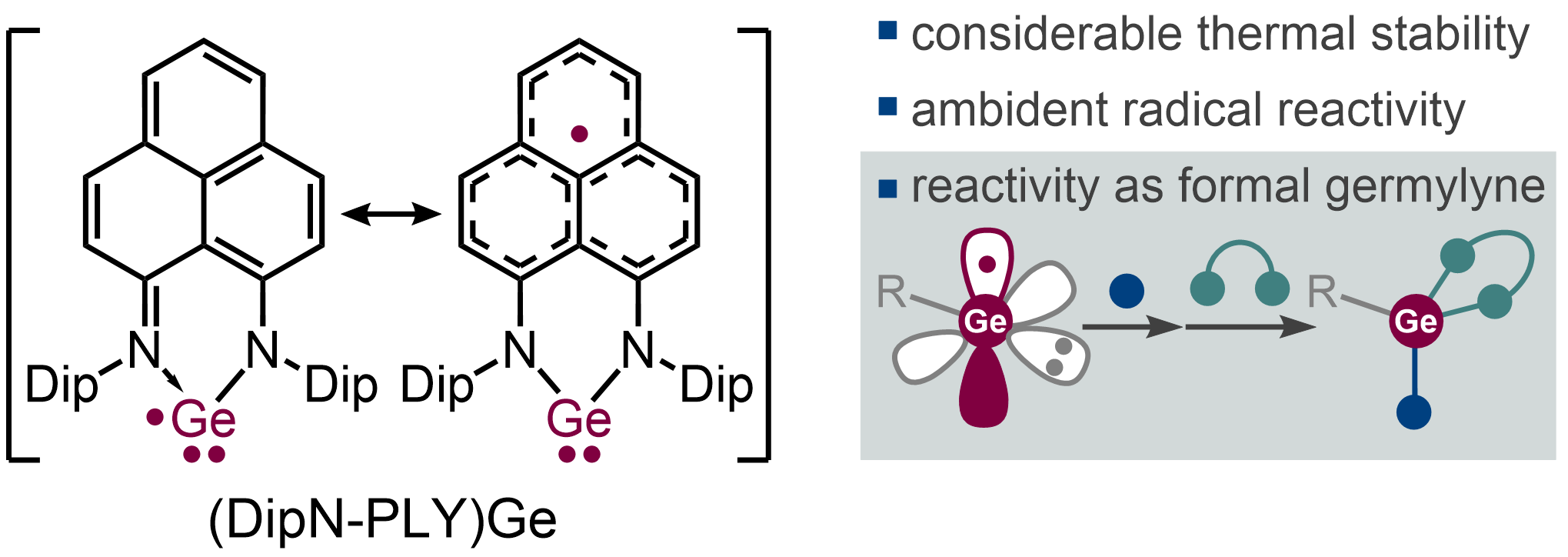
Open-Shell Germylene Stabilized by a Phenalenyl-Based Ligand
Takuya Kodama, Kenta Uchida, Chihiro Nakasuji, Ryohei Kishi, Yasutaka Kitagawa, and Mamoru Tobisu
Inorg. Chem. 2023, 62(20), 7861–7867.
DOI: https://doi.org/10.1021/acs.inorgchem.3c00583
-
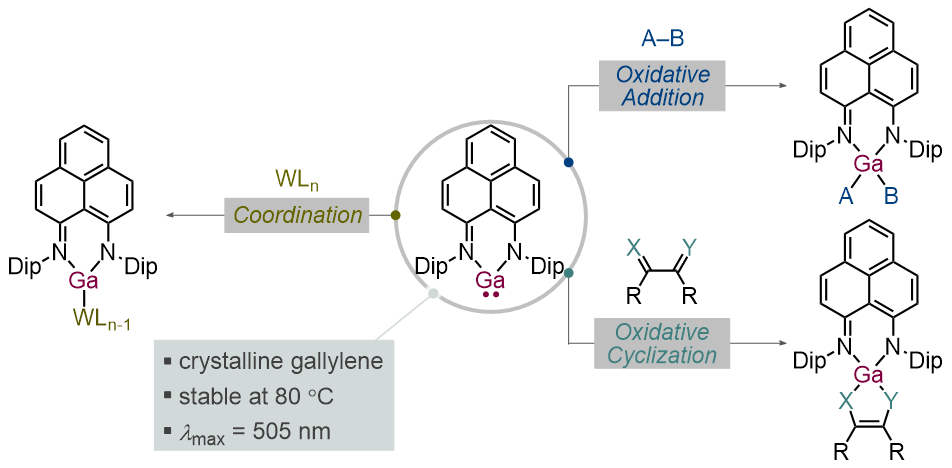
Synthesis, Structure, and Reactivity of a Gallylene Derivative Bearing a Phenalenyl-Based Ligand
Takuya Kodama, Nijito Mukai, and Mamoru Tobisu
Inorg. Chem. 2023, 62(17), 6554–6559.
DOI: https://doi.org/10.1021/acs.inorgchem.3c00697
-
「10. ヘンな有機化学反応を見つける」
鳶巣 守
有機化学イノベーション, 東京化学同人, pp. 111–122.

-
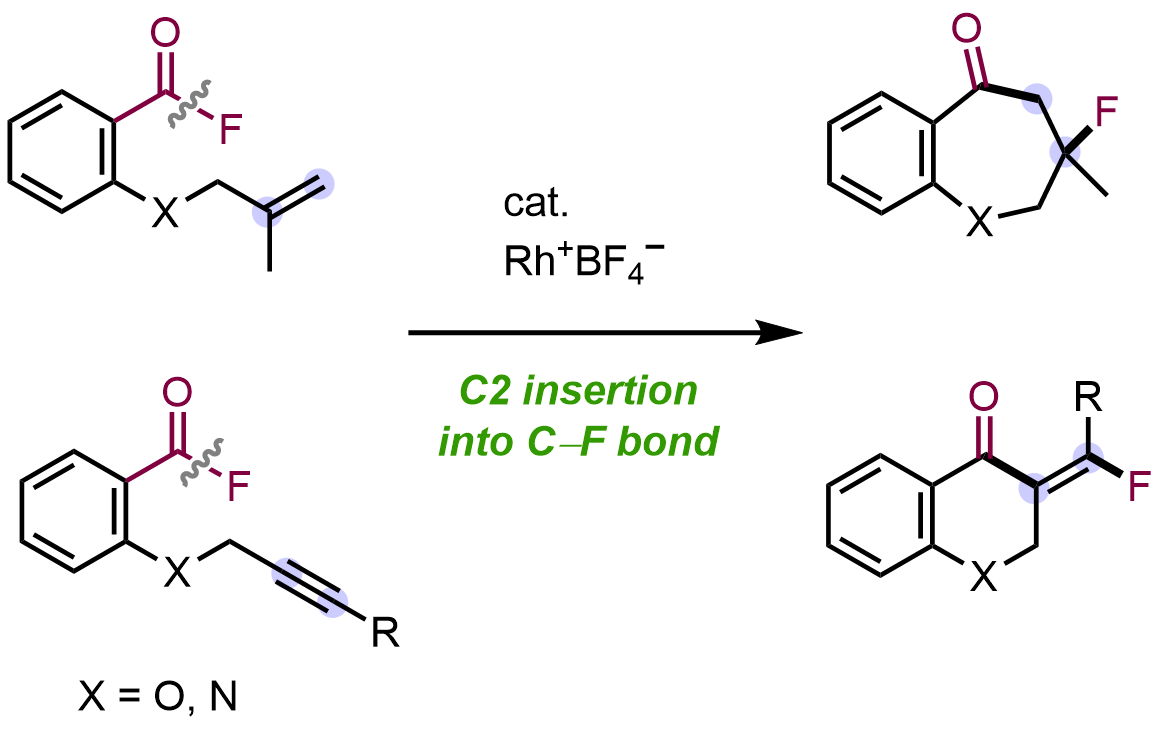
Cationic Rhodium(I) Tetrafluoroborate Catalyzed Intramolecular Carbofluorination of Alkenes via Acyl Fluoride C–F Bond Activation
Tomoki Yoshida, Masaya Ohta, Tomoya Emmei, Takuya Kodama, and Mamoru Tobisu
Angew. Chem. Int. Ed. 2023, 62(23), e202303657.
DOI: https://doi.org/10.1002/anie.202303657
-
「炭素原子一つだけを埋込む新反応 SCAD」
鳶巣 守
現代化学 2023年2月号, 東京化学同人, pp. 33–35.

-
Recent Progress in Ring-Expansion by Single Atom Editing
Hayato Fujimoto
J. Synth. Org. Chem. Jpn. 2023, 81(2), 139–140. (Review de Debut)
DOI: https://doi.org/10.5059/yukigoseikyokaishi.81.139
-
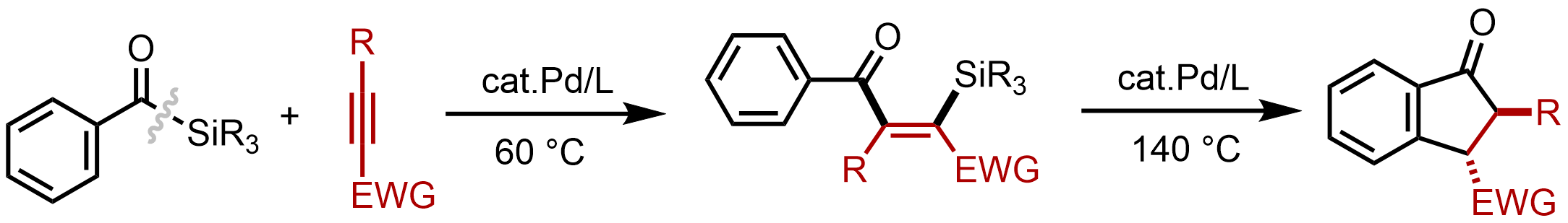
Palladium-catalyzed addition of acylsilanes across alkynes via the activation of a C–Si bond
Tetsuya Inagaki, Takahiro Ando, Shun Sakurai, Masahiro Yamanaka, and Mamoru Tobisu
Chem. Sci. 2023, 14(10), 2706–2712.
DOI: https://doi.org/10.1039/D3SC00181D
-
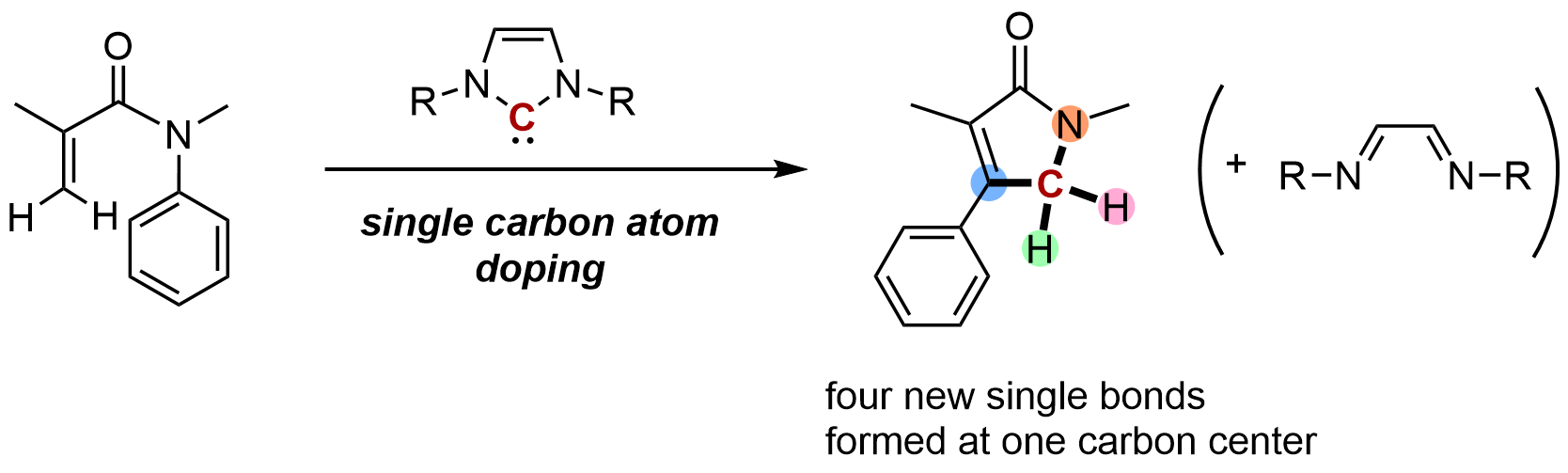
Single–carbon atom transfer to α,β-unsaturated amides from N-heterocyclic carbenes
Miharu Kamitani, Bunta Nakayasu, Hayato Fujimoto, Kosuke Yasui, Takuya Kodama, and Mamoru Tobisu
Science 2023, 379(6631), 484–488.
DOI: https://www.science.org/doi/10.1126/science.ade5110
Press release
Highlighted Science Perspective, C&EN News, Chemistry World, ASAHI Shinbun, Yahoo! News, Gendaikagaku, SynForm, Phys.org, Chem Europe, Revyuh, EurekAlert!, Sciencenewsnet.in, Bioengineer.org, Scienmag, Knowledia, Newswise, Nanowerk, Azo Life Sciences, News Azi, Bionity, etc.

-
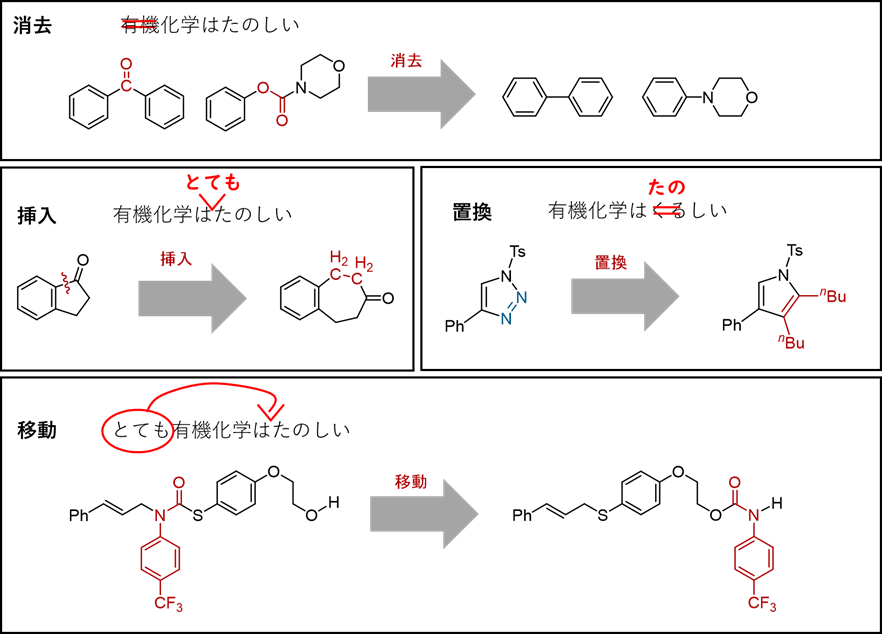
「分子を編集する」
鳶巣 守
現代化学 2023年2月号, 東京化学同人, pp. 31–34.

-
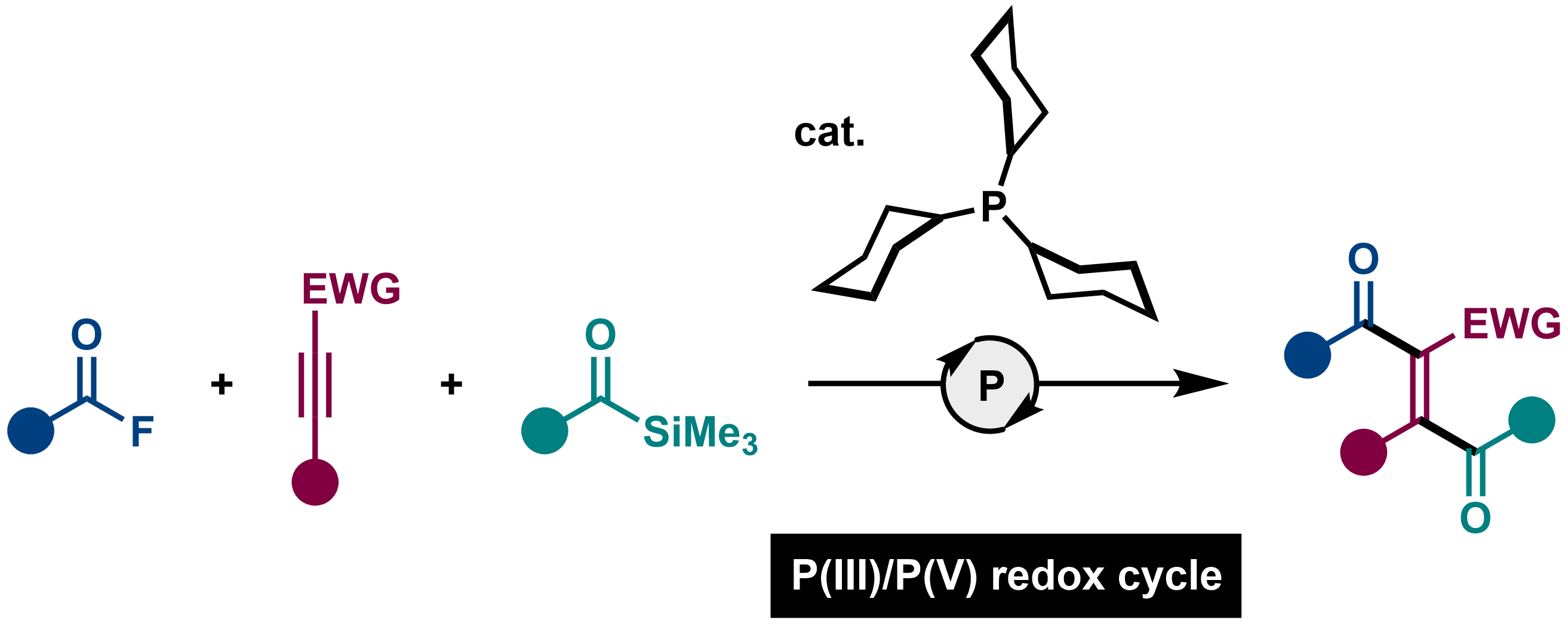
1,2-Diacylation of Alkynes Using Acyl Fluorides and Acylsilanes by P(III)/P(V) Catalysis
Hayato Fujimoto, Shisato Yamamura, Momoka Kusano, and Mamoru Tobisu
Org. Lett. 2023, 25(2), 336–340.
DOI: https://doi.org/10.1021/acs.orglett.2c03910