About TK
Takuya Kodama received his PhD degree from Osaka University in 2018 under the supervision of Prof. Takashi Kubo. He joined the group of Prof. Mamoru Tobisu at Osaka University in 2018 as an Assistant Professor. He is a member of Innovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICS-OTRI), Osaka University since 2020. He has been accepted for the FOREST (Fusion Oriented REsearch for disruptive Science and Technology) program 2023 by Japan Science and Technology Agency (JST). His main research interests are the development of organometallic and main group element compounds with π-conjugated ligand. His group has synthesized phenalenyl-based ligand and applied to main group/transition metal complexes.
He is a recipient of Chugai Pharmaceutical Co., Ltd. Award in Synthetic Organic Chemistry, Japan in 2021 and 64th Research Grant Award (UBE Industries Foundation) in 2024. He is currently a member of The Chemical Society of Japan, The Society of Synthetic Organic Chemistry, Japan, Kinka Chemical Society, Japan, and The Society of Electron Spin Science and Technology.

Research
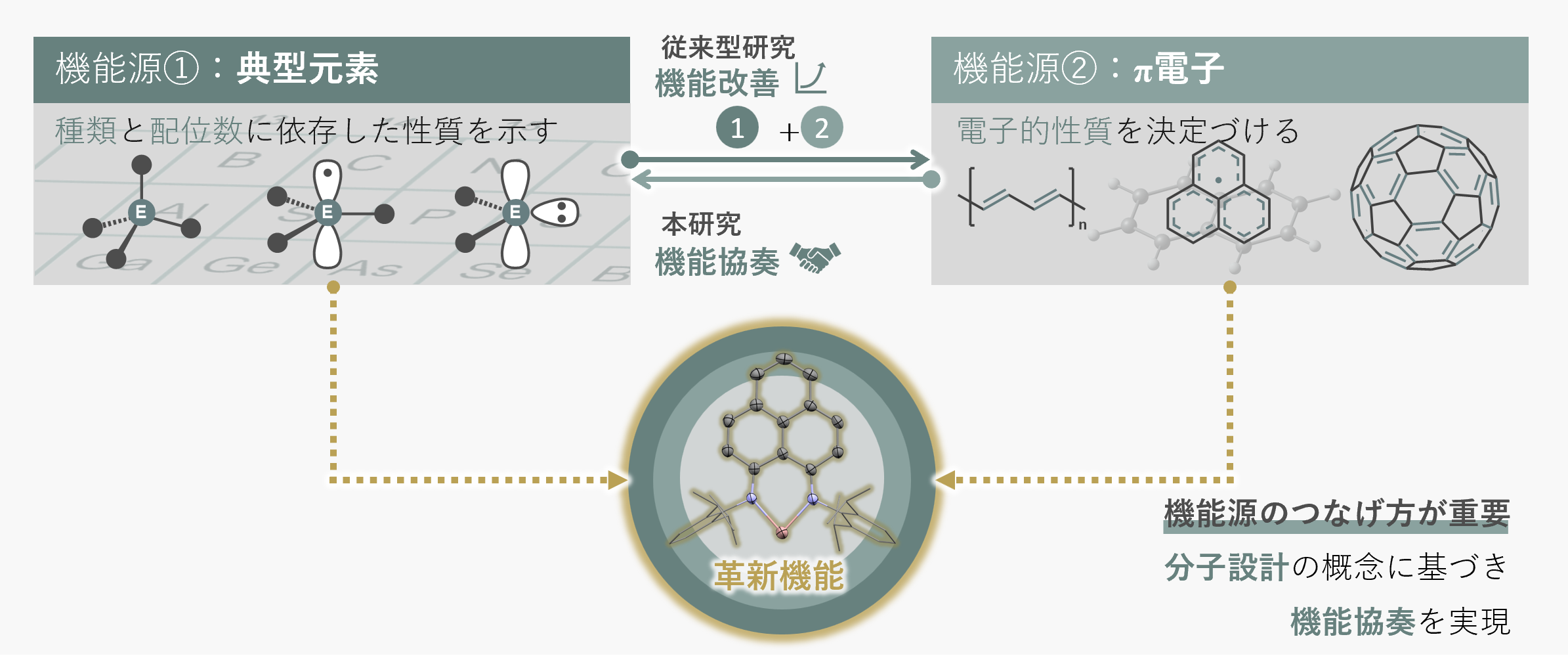
"Main group elements" and "π-electrons" are both crucial to the functionality of molecular materials. Despite the recent demand for innovative functional materials, their high potential has not been fully utilized in the field of materials science. While systems with multiple sources of functionality have been developed, these systems often operate independently or only supplement the primary function, failing to produce significant innovations.
Our research aims to explore the unexplored electronic states, physical properties, and reactivity that arise from the synergy between main group elements and π-electrons. Through this research, we seek to contribute to the creation of innovative functional materials that will benefit society in the future and establish design guidelines for these materials.
Contact
2-1 Yamada-oka, Suita, Osaka, 565-0871, JAPAN
Department of Applied Chemistry, Graduate School of Engineering, Osaka University
Assist. Prof. Takuya Kodama
E-mail: kodama "at" chem.eng.osaka-u.ac.jp "at" = @
Tel: +81-6-6879-7414
Fax: +81-6-6879-7415


